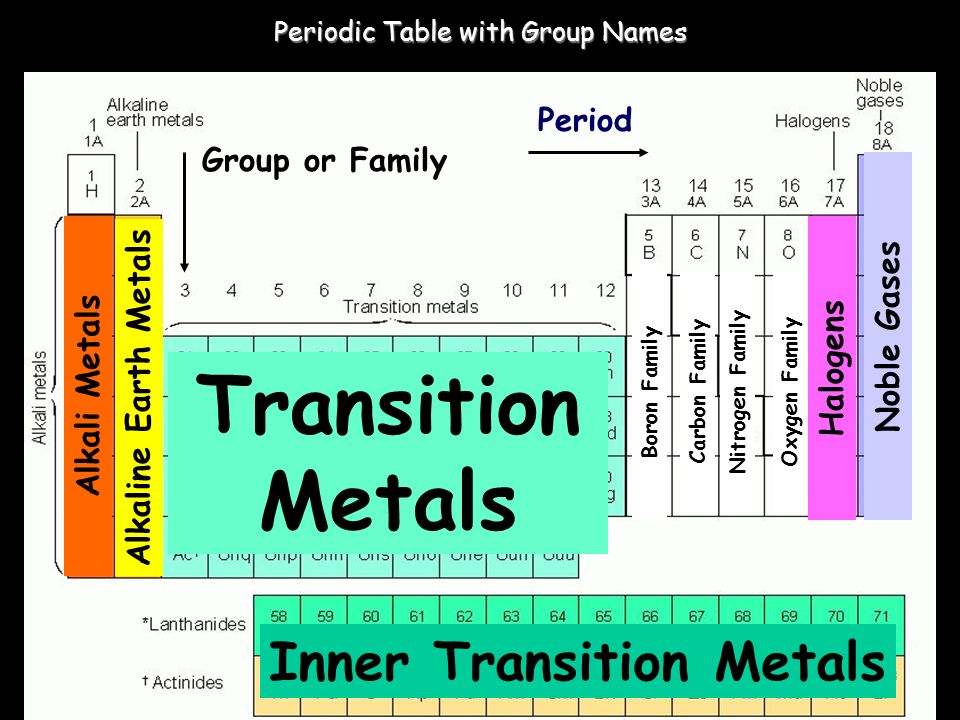
Group In The Periodic Table

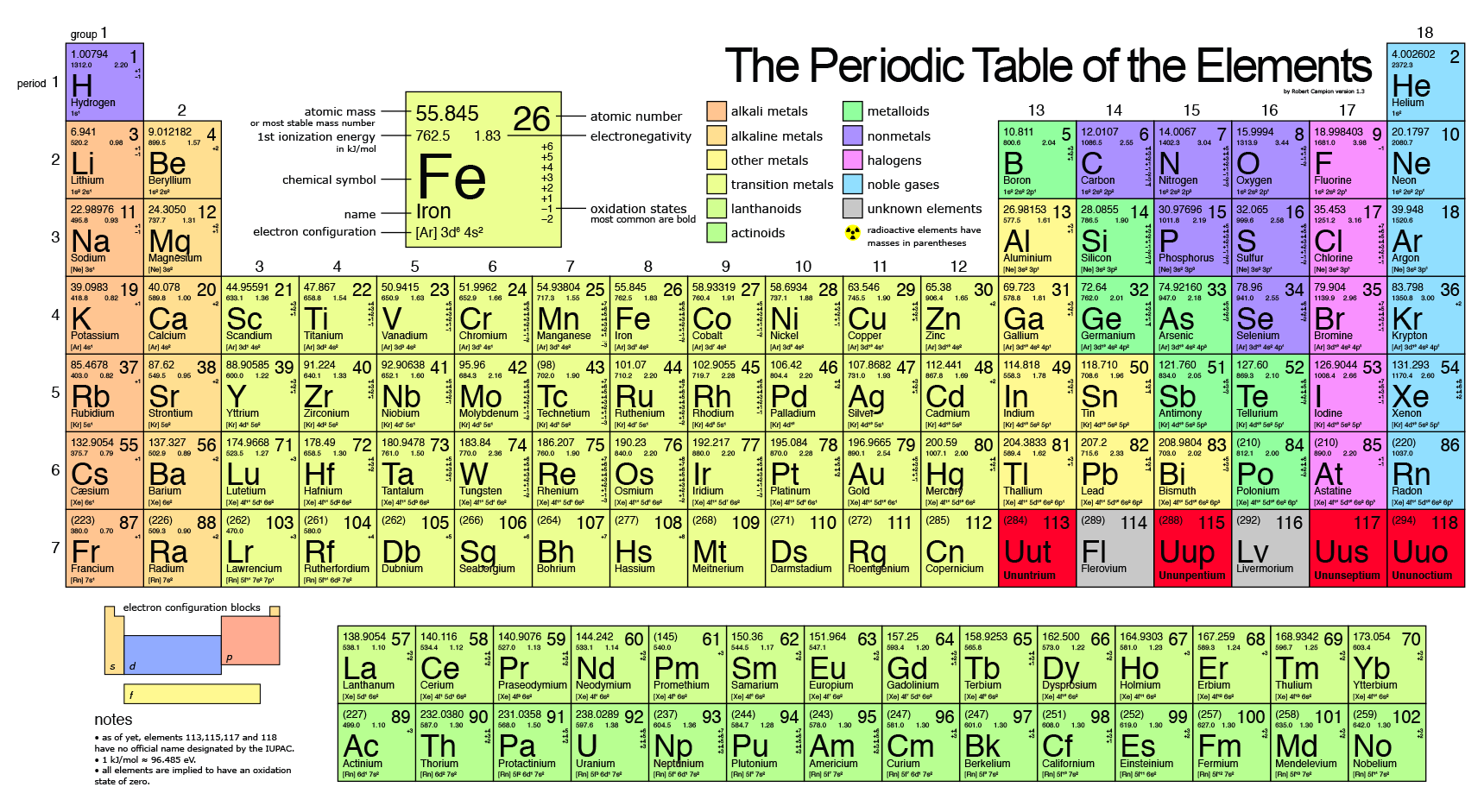
The modern periodic table, based on atomic number and electron configuration, was created primarily by a Russian chemist, Dmitri Ivanovich Mendeleev, and a German physicist, Julius Lothar Meyer, both working independently.

Interactive periodic table with dynamic layouts showing names, electrons, oxidation, trend visualization, orbitals, isotopes, and compound …
Elements as Building Blocks The periodic table is organized like a big grid. Each element is placed in a specific location because of its atomic structure. As with any grid, the periodic table has rows (left to right) and columns (up and down).
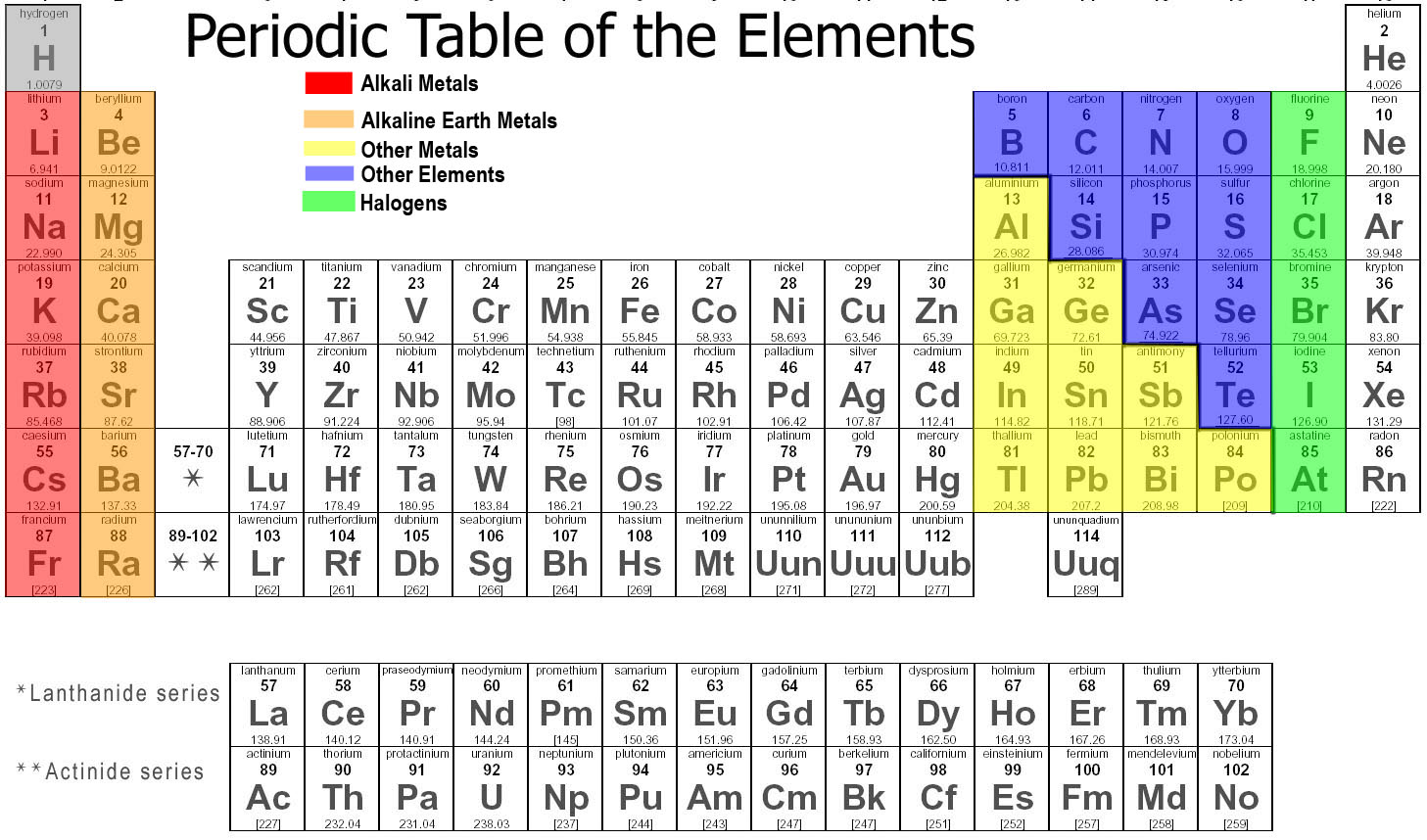
The periodic table of the elements. The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear.
In 1649, the first element was discovered through scientific inquiry by Hennig Brand; that element was phosphorous (P). The first table of the elements was published by John Newlands in Chemical News Vol. 7, Feb. 7, 1863, pp. 70-72.
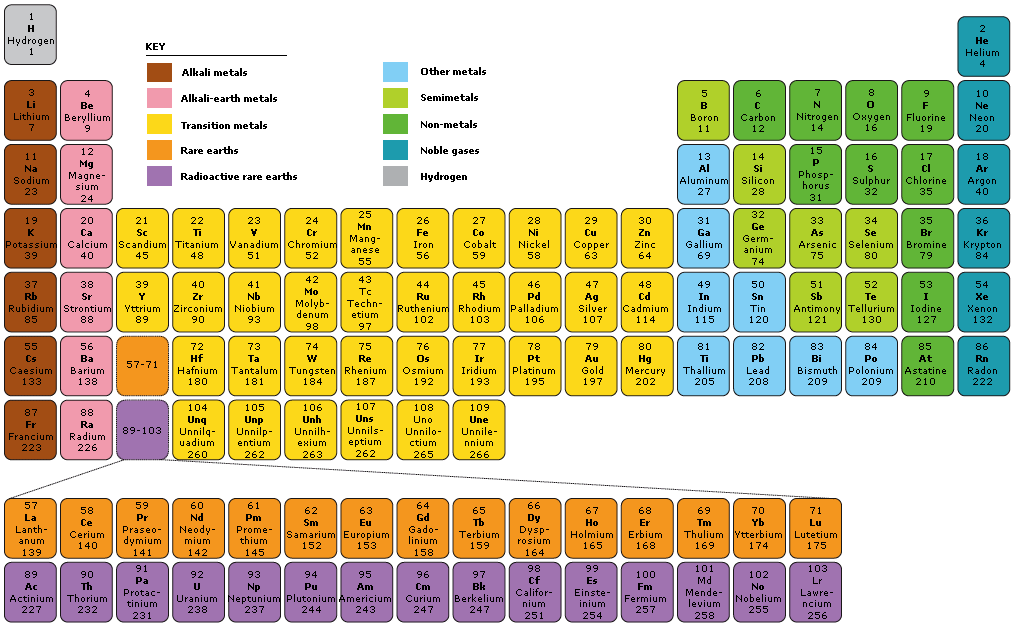
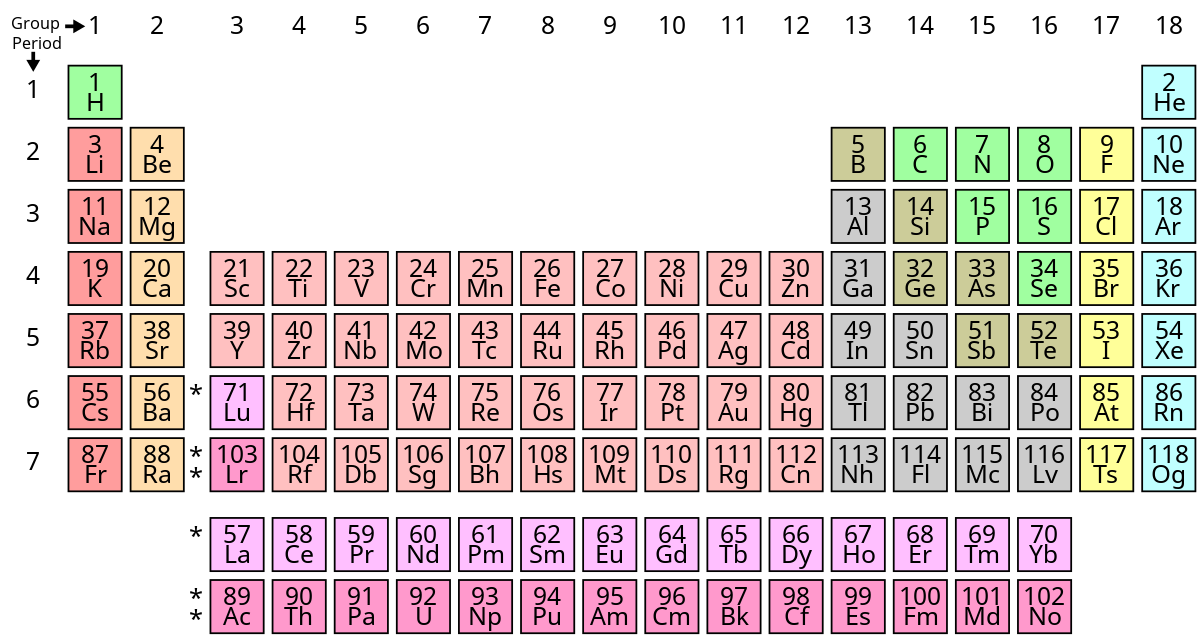
A group in the Periodic Table is any column of elements with common properties denoted by the electrons in the outermost layer. There are …

Online games. Learn about the periodic table, chemistry, the elements and more with these free online quizzes and games.
An up-to-date periodic table with detailed but easy to understand information
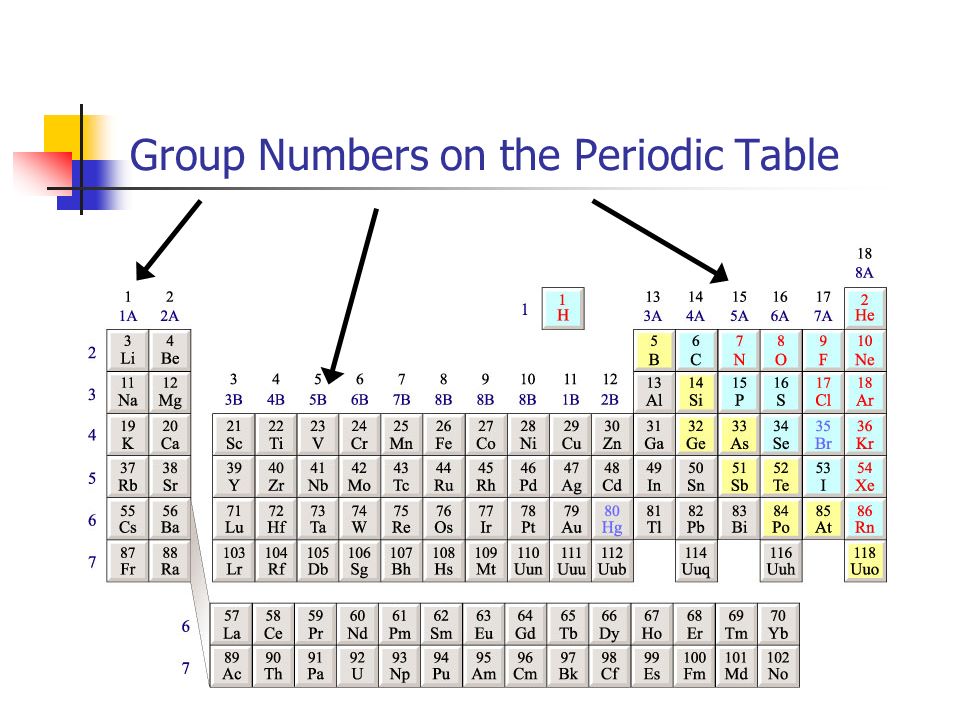
The group number is an identifier used to describe the column of the standard periodic table in which the element appears. Groups 1-2 termed s-block elements.
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configuration, and recurring chemical properties, whose adopted structure shows periodic trends.